 Location Taken: Dynamic Earth, Sudbury, ON, Canada
Location Taken: Dynamic Earth, Sudbury, ON, Canada
Time Taken: June 2010
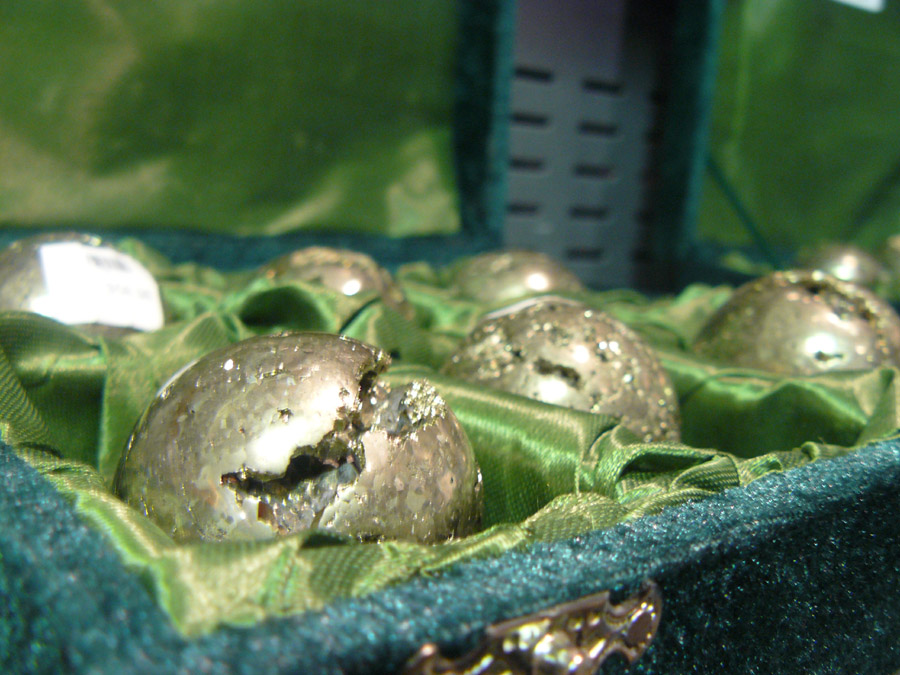
I took this photo in the gift shop at Dynamic North in Sudbury.
They’re spheres of pyrite, commonly known as fool’s gold. I guess they do look somewhat like gold, and, well, if you’ve been mining for hours in a dimly lit cave, hoping to strike your fortune, anything’s going to look like gold. Pyrite’s actually iron disulfide (one iron atom, two sulfur atoms), with the iron making it metal and the sulfur making it yellowish. Gold’s got a more yellow-orange color and native gold has a very different appearance than pyrite, running more flaky than chunky.
I find pyrite beautiful in its own right. I even prefer it over gold! Gold’s too yellow and too shiny for me. And I’m really not to big on wearing jewelry. The main thing that intrigues me about gold is its chemical properties. (It’s very big on not interacting with ANYTHING. That’s right, gold is the snobby kid of the periodic table.)
Pyrite’s much more likely to interact. It breaks down over time when exposed to air, water, and an extremophile bacteria found in the acidic conditions of abandoned mine, producing iron and sulfate. This reaction produces acid, so it starts burning away at things around it, and needs to be either neutralized or contained, or it will pollute the runoff water from the mines. It’s also a strong exothermic reaction, which means it produces heat. A lot of heat. Which means mines with a lot of pyrite have to take measures to keep the walls from spontaneously catching on fire.
Chemistry: making mine walls catch on fire since the beginning of mining.
This tendency to break down does have advantages. Some industries use it as a sulfur source, for all their acidic needs. Sulfate’s the salt version of sulfuric acid, which is the acid used in a very large array of modern industries, from fertilizers to printer ink to lubricants. And no, you can’t use it to season your food. Salt, as a generic term, is a product created by the combination of an acid and a base, forming a product with around neutral pH. These tend to be fairly stable, but water-soluble (though there are, of course, exceptions). Sodium chloride just happens to be the most common one, and the only one most people run into, so it gained the common name of salt. Well, more the other way around, really. People called Sodium chloride “salt”, and as chemistry developed, they discovered more and more compounds with salt-like attributes, and called them all salts. They tend to be rather useful compounds, really. The sulfate compound you might have heard of is Magnesium sulfate, better known as Epsom salts. It’s used for therapeutic baths. My mom uses Epsom salts all the time, to relax her muscles.
Ah, from spontaneous fires to calming baths in a few chemical transitions. Isn’t chemistry fascinating?
